Traditional Swab Collection and Transport System for Aerobic and Anaerobic Bacteria
Our Transystem™ family comprises different media for the efficient and safe transport of many bacterial strains. Choose between liquid or solid Amies and Stuart medium for aerobic culture, rapid antigen, and molecular testing; opt for gel Cary-Blair medium – with or without charcoal – for aerobic and anaerobic cultures.
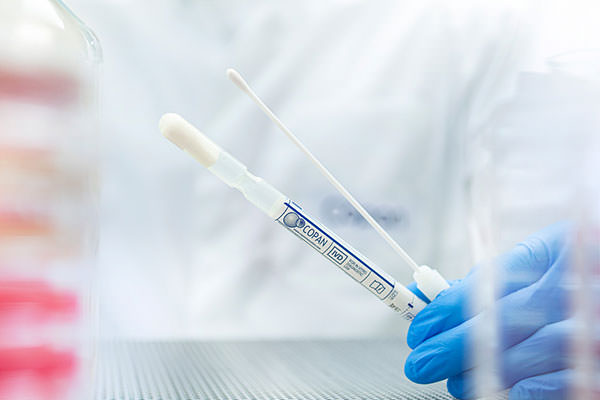
M40 Transystem™ are compliant with the CLSI M40-A standard and are available in Liquid Amies, Liquid Stuart and Amies Agar Gel with or without charcoal formulas. M40 Transystem™ applicator swabs are manufactured using a unique bio-treatment process together with a careful selection of glue and binding agents maximizing organism survival on the swab, and subsequent release into the test platform or culture media.
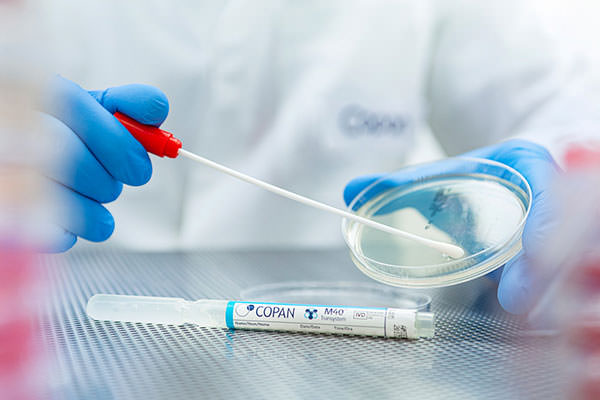
Transystem™ maintains the viability of the following organisms for 24 hours at both room and refrigerated temperature: aerobic and Anaerobic organisms – including Neisseria gonorrhoeae – Coxsackie virus B4, Herpes Simplex Virus Type 2, Influenza A, Respiratory Syncytial Virus, Varicella Zoster Virus, and Chlamydia trachomatis.

A simple, low bioburden, device for every investigation. Just uncap, collect and recap the tube!
Various swab sizes to reach different collection sites.

Choice of Liquid or solid Amies and Stuart medium for aerobic culture, rapid antigen, and molecular testing. Choice of Amies, Stuart, or Cary-Blair Gel medium (with or without charcoal), commonly used for aerobic and anaerobic cultures.

The Venturi unique “hourglass” design eliminates air pockets – harmful to anaerobe bacteria – preventing dehydration and breakdown of the gel column.

The VI-PAK metallic foil and plastic film barrier block oxygen entry, preventing unwanted oxidation of the transport medium.
This product line groups a variety of product codes, each optimized to address specific needs.
Check here the summary of these features and contact us to discover the product code that best suits your need-mix and available in your Country.
Always faithful to our scientific approach, we are eager to hear the proof of the success of our efforts and commitment.



Write us to satisfy your curiosity, get information or start a great collaboration!
Use our search tool and be surprised by how easy it is to get right to the point.